-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 6 Issue 5
Evaluation of tumor response after stereotactic body radiation therapy for lung cancer: role of 18F-FDG PET/CT
Pino Alcantara, Beatriz Cabeza Martínez, Marta García García-Esquinas, Laura G. Belaústegui, Ana Bustos
Alcantara et al. J Clin Transl Res 2020; 6(5):1
Published online: October 6, 2020
Abstract
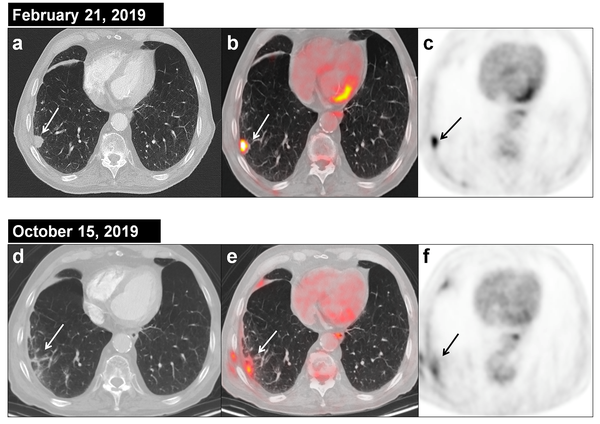
Background. Early identification of patients who fail to lung SBRT is vital as they can benefit from salvage therapy. Main guidelines recommend computed tomography (CT) to assess response and use of 18F-fluorodeoxyglucose (18F-FDG) PET/CT only when a local recurrence is suspected in CT. The pattern of radiation-induced lung injury caused by SBRT is different from changes seen after conventional radiation therapy in terms of extent, time of manifestation, and morphologic characteristics, and knowing this is crucial for proper monitoring of the tumor response. In certain cases, it may be difficult to differentiate response from progression or recurrence on CT and, in addition, some changes in CT take a long time to evolve before they are considered suspicious, making early diagnosis difficult. Metabolic changes often precede morphological changes, so 18F-FDG PET/CT quantitative and qualitative metabolic criteria can be useful in assessing early response and detecting relapses. However, the optimal practice for follow-up remains unclear and there is an active search for imaging markers for recurrent disease, including CT texture analysis, biomarker assays, new PET/CT isotopes and MRI.
Aim. To review the radiological changes that are objectified after pulmonary SBRT and the metabolic changes in 18F-FDG PET/CT, in order to assess the usefulness of following up patients with 18F-FDG PET/CT.
Relevance for patients. Currently, the evaluation of response and diagnosis of relapse after SBRT is difficult and the incorporation of routine 18F-FDG PET/CT may have value in early diagnosis of relapse when the patient may still benefit from rescue treatment.
DOI: http://dx.doi.org/10.18053/jctres.06.202005.001
Author affiliation
1. Department of Radiation Oncology. Hospital Clinico San Carlos. Madrid. Spain. Faculty of Medicine. Complutense University of Madrid. Spain
2. Department of Nuclear Medicine. Hospital Clinico San Carlos. Madrid. Spain.
3. Department of Radiology. Hospital Clinico San Carlos. Madrid. Spain. Faculty of Medicine. Complutense University of Madrid. Spain.
*Corresponding author
Pino Alcantara
Department of Radiation Oncology. Hospital Clinico San Carlos. Madrid. Spain. Faculty of Medicine. Complutense University of Madrid. Spain.
Email: mariapino.alcantara@salud.madrid.org; pinoac2@gmail.com; malcanta@ucm.es
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

