-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 9 Issue 6
Biocompatibility of calcitonin receptor fragment peptide-treated 3D-printed bone scaffolds: a muscle pouch implantation study
Vamiq M. Mustahsan, Yanming Cai, David E. Komatsu, Imin Kao, Srinivas Pentyala*
Mustahsan et al. J Clin Transl Res 2023; 9(6):23.00097
Published online: November 18, 2023
Abstract
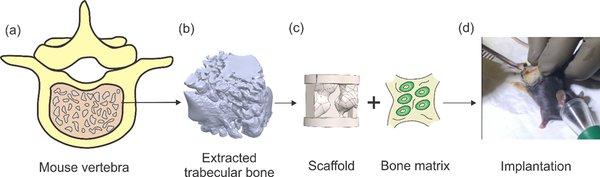
Background and aim: Current synthetic bone graft substitutes (BGS) in development are limited by high resorption, poor load-bearing properties, and stress shielding. These limitations inhibit BGS from complete bio-integration. In this study, we developed calcitonin receptor fragment peptide (CRFP)-treated non-biodegradable MED610 scaffold, seeded with MC3T3 stem cells, and assessed their in vivo biocompatibility and bio-integration.
Methods: Scaffolds were fabricated with Stratasys MED610 (MED610) material, seeded with Mus musculus calvaria cells (MC3T3) and osteogenesis was induced with CRFP after the cells reached confluency and generated bone matrix. Scaffolds with and without bone matrix were implanted in male mice following a muscle pouch implantation protocol. Post-extraction, imaging, staining, and mechanical compression testing was carried after 3 weeks of scaffold implantation in the muscle to measure the ectopic bone formation and compressive strength.
Results: The implanted scaffolds showed significantly higher (p < 0.01) calcium deposits in comparison to the untreated scaffolds. We also found significantly higher (p < 0.001) mineralization on the implanted scaffolds compared to scaffolds before implantation. The mechanical properties of the scaffolds did not vary significantly.
Conclusions: MED610 scaffolds treated with CRFP in vivo do not cause any adverse reaction when implanted in muscle and showed significant ectopic bone formation, indicating biocompatibility and bio-integration.
Relevance for patients: This study will aid in developing biomimetic and biocompatible artificial bones for implantation.
DOI: http://dx.doi.org/10.18053/jctres.09.202306.23-00097
Author affiliation
1. Department of Anesthesiology, Stony Brook University Renaissance School of Medicine, Stony Brook, 11794 NY, United States of America
2. Department of Orthopaedics & Rehabilitation and Stony Brook University Renaissance School of Medicine, Stony Brook, 11794 NY, United States of America
3. Department of Mechanical Engineering Stony Brook University College of Engineering and Applied Sciences, Stony Brook, 11794 NY, United States of America
*Corresponding author
Srinivas Pentyala
Anesthesiology, HSC L4, Room:060, Renaissance School of Medicine, Stony Brook, NY 11794-8480, United States of America.
Tel: +1 631-444-2974
Fax: +1 631-444-2907
Email: Srinivas.pentyala@stonybrook.edu
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Chemistry, Utrecht University, Utrecht, the Netherlands
Department of Pathology, Erasmus Medical Center, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

