-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 9 Issue 1
No difference in treatment outcome between patients with nodal versus extranodal diffuse large B-cell lymphoma
Saqib Raza Khan*, Muhammad Afzal, Salman Muhammad Soomar, Daania Shoaib, Ayesha Arshad Ali, Muhammad Tariq, Muhammad Nauman Zahir, Adnan Abdul Jabbar, Yasmin Abdul Rashid, Michal Heger, Munira Shabbir Moosajee
Khan et al. J Clin Transl Res 2023; 9(1):5
Published online: December 28, 2022
Abstract
Background and aim: Diffuse large B-cell lymphoma (DLBCL) has been classified using various parameters, including the site of origin. Studies have reported conflicting outcomes when DLBLC patients were stratified according to the site of origin. This study aimed to investigate the response rate and survival outcomes in nodal versus extranodal DLBCL and compare the results to a region-matched study covering the 1988-2005 period.
Methods: A single-center retrospective cohort study was conducted on all patients diagnosed with DLBCL and treated in a tertiary care hospital in Pakistan during 2014-2019. We calculated the mean and median for continuous variables and frequency and percentages for all categorical variables. Progression-free survival (PFS) and overall survival (OS) were calculated using Kaplan-Meier survival curves. A Cox proportional hazards model was used to determine the hazard ratio (HR) for OS.
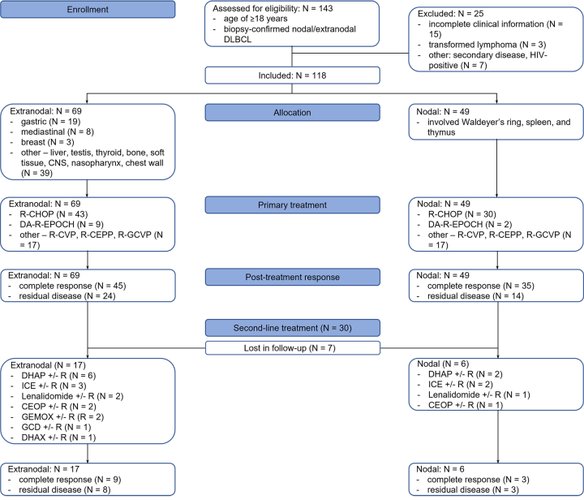
Results: Of the 118 patients, 49 patients (41.5%) had nodal disease and 69 patients (58.5%) were diagnosed with extranodal DLBCL. The majority of patients in the nodal and extranodal cohorts presented with stage III and IV disease (73.4% and 62.3%, respectively). A complete response to (immuno)chemotherapy was achieved in 71.4% of nodal DLBCL patients and 65.2% of extranodal DLBCL patients. The 5-y PFS and median PFS in the entire cohort were 0.8% and 17 m, respectively. The PFS and median PFS in the nodal and extranodal DLBCL cohort were 0% and 1.4%, respectively, and 15 m and 19 m, respectively. The 5-y OS and median OS in the entire cohort were 16.1% and 19 m, respectively. The OS and median OS in the nodal and extranodal DLBCL cohort were 8.2% and 21.7%, respectively, and 19 m and 21 m, respectively. Multivariable linear regression revealed that the ABC phenotype (nodal, HR = 1.37, 95% CI = 1.37-3.20; extranodal, HR = 1.65, 95% CI = 1.46-3.17; GBC as reference) and double and triple hit DLBCL (nodal, HR = 1.29, 95% CI = 1.19-2.81; extranodal, HR = 1.87, 95% CI = 1.28-2.43; non-expressors as reference) are independent negative predictors of OS.
Conclusions: DLBCL incidence in the Karachi region has remained comparable but patient composition in the extranodal DLBCL cohort has shifted to predominantly advanced stage. Nodal and extranodal DLBCL were associated with similar PFS and OS profiles and first- and second-line treatment responses. Cell of origin and antigen expression status were independent negative predictors of OS, disfavoring the ABC phenotype and lesions with c-MYC and BCL2 and/or BCL6 overexpression.
Relevance for patients: DLBCL is an aggressive type of non-Hodgkin's lymphoma, however; patients respond well to standard systemic chemotherapy. Extranodal type of DLBCL patients tend to have more residual disease after first-line systemic chemotherapy, but physicians should keep in mind that the subsequent line treatment mitigates its negative impact on survival
DOI: http://dx.doi.org/10.18053/jctres.09.202301.005
Author affiliation
1. Department of Oncology, Aga Khan University Hospital, Karachi, Pakistan
2. Medical College, Aga Khan University, Karachi, Pakistan
3. Lady Reading Hospital, Peshawar, Pakistan
4. Department of Pharmaceutics, Jiaxing Key Laboratory for Photonanomedicine and Experimental Therapeutics, College of Medicine, Jiaxing University, Jiaxing, Zhejiang, P. R. China
5. Laboratory Experimental Oncology, Department of Pathology, Erasmus MC, Rotterdam, the Netherlands
6. Membrane Biochemistry and Biophysics, Department of Chemistry, Faculty of Science, Utrecht University, Utrecht, the Netherlands
*Corresponding author
Saqib Raza Khan
Department of Oncology, Aga Khan University Hospital, Karachi, Pakistan.
Email: saqib.raza31@yahoo.com
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Chemistry, Utrecht University, Utrecht, the Netherlands
Department of Pathology, Erasmus Medical Center, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

