-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 8 Issue 6
Endoluminal vacuum therapy for rectal anastomosis is safe and does not increase risk of strictures in a swine model
Alexander Ostapenko†, Shawn Liechty†, Daniel Kleiner*
Ostapenko et al. J Clin Transl Res 2022; 8(6):1
Published online: October 7, 2022
Abstract
Background: Endoluminal vacuum therapy has been experimentally used in patients with esophageal, rectal, and Roux-en-Y bypass surgery. Yorkshire pigs are good animal models for studying the safety and efficacy of endoluminal vacuum therapy and prior studies have employed these devices in rectal anastomotic defects, as rescue therapy for early anastomotic leaks, as well as prophylactic therapy as a means of protecting high risk anastomosis.
Aim: The objective of this study is to assess the effects of a prophylactic vacuum assist device on bowel tissue surrounding an intact anastomosis at 30 days post device removal.
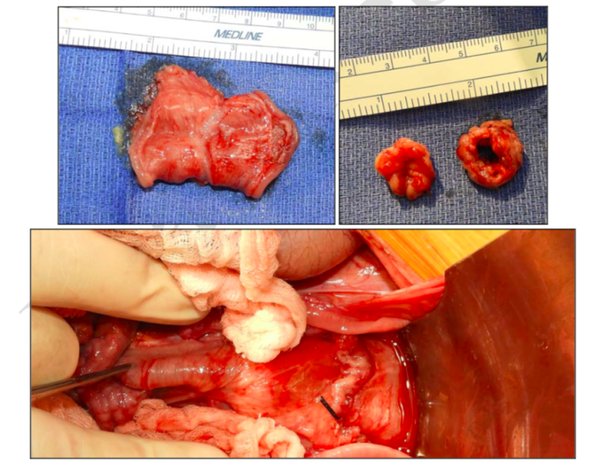
Methods: A total of seven pigs underwent a rectal resection with primary anastomosis: five experimental pigs with a prophylactic endoluminal vacuum device in place for five days post surgery and two control pigs with no device. All animals were euthanized on the 35th post-operative day and subjected to a necropsy with a histopathological evaluation of the rectal anastomosis.
Results: No significant difference in inflammation or strictures was observed between the anastomosis of animals with the endoluminal vacuum devices and controls.
Conclusion: We therefore conclude that endoluminal vacuum therapy is safe for prophylactic use in pigs undergoing low anterior resection and does not cause significant strictures.
Relevance for patients: Anastomotic leak is a feared complication resulting in increased costs, length of stay, and emotional distress. Endoluminal negative pressure vacuum therapy is a new technology that has been used in experimental models in both animals and humans for prevention and treatment of anastomotic leak. In this series we demonstrate endoluminal vacuum therapy is safe in a porcine model and does not result in stricture or increased adhesion formation. We expect endoluminal vacuum therapy to become more widely used in the future in both prevention and treatment of anastomotic leaks.
†These authors contributed equally to this work.
DOI: http://dx.doi.org/10.18053/jctres.08.202206.001
Author affiliation
1. Department of General Surgery, Nuvance Health, CT, USA
2. Department of General Surgery, Waterbury Hospital, CT, USA
*Corresponding author
Daniel Kleiner
Department of Surgery, Waterbury Hospital, 64 Robbins St, Waterbury, CT 06708, USA
Tel: +1 203-981-2981
Email: danieleduardkleiner@gmail.com
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

