-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 8 Issue 5
Identifying critically ill patients with cirrhosis who benefit from nutrition therapy: the mNUTRIC score study
Harshita Tripathi, Jaya Benjamin*, Rakhi Maiwall, Puneet Puri, Puja Bhatia Kapoor, Varsha Shasthry, Vandana Saluja, Prashant Agrawal, Guresh Kumar, Yogendra Kumar Joshi, Shiv Kumar Sarin
Tripathi et al. J Clin Transl Res 2022; 8(5):9
Published online: September 13, 2022
Abstract
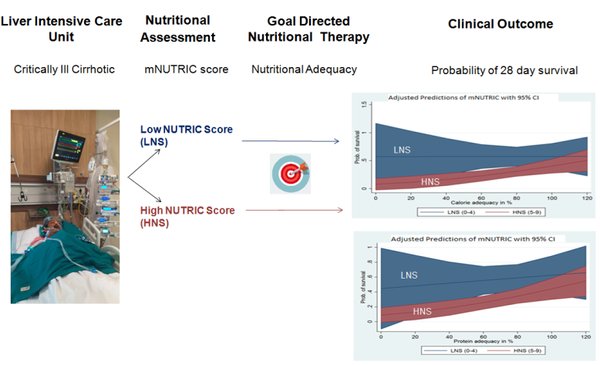
Background and aim: Malnutrition increases risk of mortality in critically ill patients with cirrhosis. Modified Nutrition Risk in Critically ill (mNUTRIC) score is a validated tool to identify at risk patients who may benefit from goal-directed nutrition therapy. We aimed to study the association between mNUTRIC score and 28-day mortality in critically ill patients with cirrhosis.
Methods: A prospective study was conducted in the liver intensive care unit of a quaternary teaching institute. Baseline and follow-up data pertaining to mNUTRIC score, clinical-, hemodynamic-, biochemical-, nutritional parameters, mechanical ventilation, length of ICU stay, and development of sepsis were collected. Correlation between mNUTRIC score and its modulation by nutritional adequacy was determined.
Results: One hundred and fifty patients were enrolled. Out of these, 116 (77%) had a high NUTRIC score (HNS) and 34 (23%) had a low NUTRIC score (LNS). Patients with HNS had higher mortality (54% vs. 10%; p = 0.008), longer mechanical ventilation (p = 0.02), and high incidence of sepsis (32% vs. 2.6%; p = 0.002) compared to LNS. The probability of survival increased with increase in nutritional adequacy (p < 0.01) in patients with HNS.
Conclusion: mNUTRIC score is a useful tool for identifying nutrition risk in critically ill patients with cirrhosis. Goal-directed nutrition therapy in patients with HNS can significantly improve survival.
Relevance for patients: Critically ill patients with cirrhosis who are at a higher nutritional risk as identified by the mNUTRIC score may have a better survival benefit if higher calorie and protein adequacy is achieved in the ICU.
DOI: http://dx.doi.org/10.18053/jctres.08.202205.009
Author affiliation
1. Department of Clinical Nutrition, Institute of Liver and Biliary Sciences, New Delhi, India
2. Department of Hepatology, Institute of Liver and Biliary Sciences, New Delhi, India
3. Section of Gastroenterology, Hepatology and Nutrition, Hunter Holmes McGuire VA,
Medical Center, Virginia Commonwealth University, Richmond VA, United States
4. Department of Anesthesia and Critical Care, Institute of Liver and Biliary Sciences, New Delhi, India
5. Department of Clinical Research, Institute of Liver and Biliary Sciences, New Delhi, India
*Corresponding author
Jaya Benjamin
Department of Clinical Nutrition, Institute of Liver and Biliary Sciences, New Delhi, India
Tel: +91 9540951081
Email: jayabenjaminilbs@gmail.com
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

