-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 8 Issue 4
Assessment of angiogenic potential of mesenchymal stem cells derived conditioned media from various oral sources
Madhura Rajendra Shekatkar, Supriya Mohit Kheur*, Avinash Haribhau Kharat, Shantanu Sanjeev Deshpande, Avinash Purushottam Sanap, Mohit Gurunath Kheur, Ramesh Ramchandra Bhonde
Shekatkar et al. J Clin Transl Res 2022; 8(4):7
Published online: July 25, 2022
Abstract
Background: Abnormal angiogenesis hamper blood vessel proliferation implicated in various biological processes. The current method available to clinically treat patients to enhance angiogenesis is administering the angiogenic growth factors. However, due to a lack of spatiotemporal control over the substantial release of these factors, numerous drawbacks are faced such as leaky vasculature. Hence stem-cell-based therapeutic applications are running their race to evolve as potential targets for deranged angiogenesis. In clinical dentistry, adequate tissue vascularisation is essential for successful endodontic therapies like apexogenesis and apexification. Also, wound healing of the extraction socket and tissue regeneration post-surgical phase of treatment including implant placement, require angiogenesis as a foundation for the ultimate success of treatment. Mesenchymal stem cells (MSCs) secrete certain growth factors and cytokines in the culture medium during the proliferation. These factors and cytokines are responsible for various biological activities inside our bodies. Oral cavity-derived stem cells can secrete growth factors that enhance angiogenesis.
Aim: To investigate the angiogenic potential of conditioned media (CM) of mesenchymal stem cells derived from different oral sources.
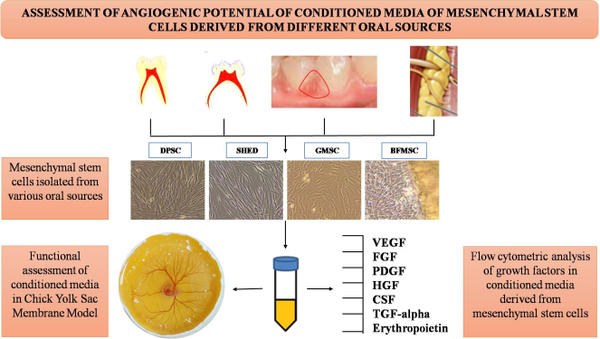
Methods: Oral tissues such as dental pulp of adult and deciduous teeth, gingiva and buccal fat were used to isolate dental pulp MSCs (DPSCs), exfoliated deciduous teeth (SHED), gingival MSCs (GMSCs) and buccal fat derived MSCs (BFMSCs). MSCs conditioned medium (CM) from passage 4 cells from all the sources were obtained at 48 h interval, and growth factor analysis was performed using flow cytometry. To assess the functionality of the CM, Chick Yolk Sac Membrane (YSM) assay was performed.
Results: CM obtained from DPSCs showed higher levels of vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF) and hepatocyte growth factor (HGF) as evidenced by flow cytometry. Furthermore, DPSC-CM exhibited significantly higher pro-angiogenic potential when assessed in in- ovo YSM assay.
Conclusion: DPSCs so far seems to be the best source as compare to the rest of oral sources in promoting angiogenesis. A novel source of CM derived from buccal fat stem cells was used to assess angiogenic potential. Thus, the present study shows that CM derived from oral cavity-derived -MSCs has a dynamic and influential role in angiogenesis.
Relevance for patients: CM derived from various oral sources of MSCs could be used along with existing therapies in medical practice where patients have compromised blood supply like in diabetes and in patients with debilitating disorders. In clinical dentistry, adequate tissue vascularisation is essential for successful wound healing, grafting procedures and endodontic therapies. DPSCs-CM shows better angiogenic potential in comparison with other oral sources of MSCs-CM. Our findings could be a turning point in management of all surgical and regenerative procedures requiring increased angiogenesis.
DOI: http://dx.doi.org/10.18053/jctres.08.202204.007
Author affiliation
1. Department of Oral Pathology and Microbiology, Dr. D. Y. Patil Dental College and Hospital, Dr. D. Y. Patil Vidyapeeth, Pimpri, Pune, India.
2. Regenerative Medicine Laboratory, Dr. D. Y. Patil Dental College and Hospital, Dr. D. Y. Patil Vidyapeeth, Pimpri, Pune, India.
3. Department of Pediatric and Preventive Dentistry, Terna Dental College and Hospital, Navi Mumbai, India.
4. Department of Prosthodontics, M.A. Rangoonwala College of Dental Sciences and Research Centre, Pune, India.
*Corresponding author
Supriya Kheur
Department of Oral Pathology and Microbiology, Dr. D. Y. Patil Dental
College and Hospital, Dr. D. Y. Patil Vidyapeeth, Pimpri, Pune, India.
Regenerative Medicine Laboratory, Dr. D. Y. Patil Dental College and
Hospital, Dr. D. Y. Patil Vidyapeeth, Pimpri, Pune, India.
Tel: +91-9970150760
Email: supriya.kheur@dpu.edu.in
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

