-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 7 Issue 6
Definitive chemoradiation in locally advanced inoperable esophageal cancer patients – Retrospective analysis of different chemotherapy regimens in a tertiary cancer centre
Aswin Nagarajan*, Begum Yesmin Nureja, Ramya Ravichandar, Rama Ranganathan
Nagarajan et al. J Clin Transl Res 2021; 7(6):3
Published online: October 30, 2021
Abstract
Background and aim: Definitive chemoradiation (dCRT) is the standard treatment for locally advanced inoperable esophageal cancer. The aim of this study is to analyze the effect of dCRT combined with paclitaxel, and carboplatin (TC) against cisplatin (CDDP) with radiation.
Methods: The study population included patients with locally advanced inoperable esophageal cancer seeking treatment at our center from March 2013 to December 2017. Case records from 66 patients were extracted. The toxicity profile of patients who received TC or CDDP was reported and analyzed. A chi-square test and students t-test were used to analyze the categorical, and the continuous variables, respectively. The Kaplan-Meier method was used to estimate the survival probability. A log rank test was applied to compare the survival differences between the two groups.
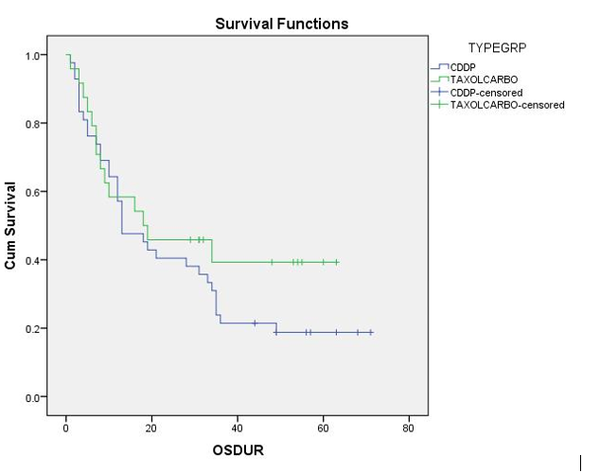
Results: The overall survival (OS) did not differ at 3 years between the TC and CDDP (p-value = 0.286). The median survival duration was 13 months for CDDP and 18 months for TC. The toxicity profile like emesis (93% CDDP vs 25% TC), neutropenia (79% CDDP vs 13% TC), thrombocytopenia (10% CDDP vs 17% TC) and dyselectrolytemia (71% CDDP vs 8% TC) were compared between the two treatment groups and found to be more in CDDP group.
Conclusion: The treatment of patients with locally advanced esophageal carcinoma with dCRT and TC showed an improved toxicity profile, but similar OS compared to CDDP. Applying dCRT with TC could be an alternate regimen for locally advanced inoperable esophageal cancer patients.
Relevance for patients: Concurrent chemoradiation with paclitaxel and carboplatin regimen can be considered as an alternative for cisplatin as it shows equivalent survival and reduced toxicity profile.
DOI: http://dx.doi.org/10.18053/jctres.07.202106.003
Author affiliation
1. Department of Radiation oncology, Cancer Institute, Adyar, Chennai, Tamil Nadu, India.
2. Department of Pharmacology, Sree Balaji Medical College,Chennai, Tamil Nadu, India.
3. Department of Epidemiology, Biostatistics and Cancer Registry, Cancer Institute, Adyar, Chennai, Tamil Nadu, India.
*Corresponding author
Aswin Nagarajan
Department of Radiation oncology, Cancer Institute, Adyar, Chennai, Tamil Nadu, India
Email: ashwinnagu@rediffmail.com
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

