-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 7 issue 2
Association of losartan with outcomes in metastatic pancreatic cancer patients treated with chemotherapy
Anup Kasi#*, Jessica Allen#, Kathan Mehta, Prasad Dandawate, Subhrajit Saha, Stefan Bossmann, Shrikant Anant, Weijing Sun
Kasi et al. J Clin Transl Res 2021; 7(2):8
Published online: March 24, 2021
Abstract
Background: Previous trials have shown improved efficacy of neoadjuvant treatment when combined with angiotensin II receptor antagonist, losartan in patients with locally advanced pancreatic ductal adenocarcinoma (PDA). However, role of losartan is unknown in metastatic PDA. In this retrospective observational study, we examined the relationship between losartan use at time of diagnosis and continued through chemotherapy treatment with clinical outcomes in patients with metastatic PDA that received chemotherapy.
Methods: We retrospectively evaluated 114 metastatic PDA patients treated at University of Kansas Cancer Center between January 2000 and November 2019. We compared overall survival (OS), progression free survival (PFS), objective response rate (ORR), and disease control rate (DCR) between patients using losartan at time of their cancer diagnosis and a control group of patients who were not on losartan. A subgroup analysis was performed based on patients who were on a 100 mg dose of losartan along with chemotherapy versus versus patients treated with chemotherapy (without losartan). Another subgroup analysis was performed based on chemotherapy regimen: FOLFIRINOX versus Gemcitabine and Abraxane.
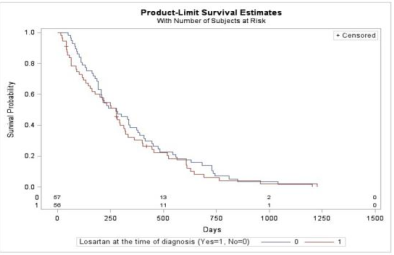
Results: Table 1 shows baseline demographics of the study. No significant difference was found in OS [p=0.466] or PFS [p=0.919] in patients on losartan (median 274d, 83d) and control patients (median 279d, 111d). No significant difference was found in ORR [p=0.621] or in DCR [p=0.497]. No significant difference was found in OS [p=0.771] or PFS [p=0.0604] in losartan patients (median 347d, 350d) and control patients (median 333d, 101d) treated with FOLFIRINOX. No significant difference was found in OS [p=0.916] or PFS [p=0.341] in losartan (median 312d, 69d) and control patients (median 221d, 136d) treated with Gemcitabine plus Abraxane. No significant difference was found in OS [p=0.727] or PFS [p=0.790] in 100mg losartan patients (median 261d, 84d) and control (median 279d, 111d).
Conclusions: Patients on losartan at time of diagnosis and continued through chemotherapy treatment had no significant difference in OS, PFS, ORR, DCR than control patients. Subgroup analysis of patients treated with FOLFIRINOX revealed a longer PFS with losartan than control but did not reach statistical significance, likely due to small sample size. Our findings should be validated in a larger cohort to confirm if the benefit of losartan and FOLFIRINOX seen in a neoadjuvant setting for locally advanced cancer also applies to metastatic cancer.
Relevance for Patients: This research adds to growing data on the efficacy of angiotensin receptor blocking drugs as adjunctive treatment in addition to chemotherapy in pancreatic cancer with specific focus on metastatic disease.
DOI: http://dx.doi.org/10.18053/jctres.07.202102.008
# These authors contributed equally to this work
Author affiliation
1. University of Kansas Medical Center Division of Medical Oncology, Kansas, USA
2. University of Kansas School of Medicine, Kansas, USA
3. University of Kansas School of Medicine Division of Cancer Biology, Kansas, USA
4. University of Kansas School of Medicine Division of Radiation Oncology, Kansas, USA
*Corresponding author
Anup Kasi
University of Kansas Medical Center Division
of Medical Oncology, Kansas, USA
Email: akasi@kumc.edu
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

