-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 6 Issue 2
Acetaminophen-induced apoptosis: facts versus fiction
Hartmut Jaeschke, Anup Ramachandran
Jaeschke and Ramachandran, J Clin Transl Res 2020, 6(2):2
Published online: August 1, 2020
Abstract
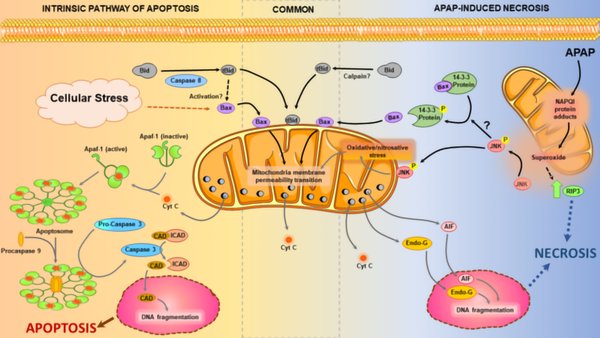
An overdose of the widely used analgesic acetaminophen (APAP) is the most common cause of acute liver failure in the western world and hence is a clinically significant problem. Thus, mechanisms of APAP-induced hepatotoxicity have been the focus of extensive investigation for decades and it was established that APAP induces hepatocyte cell death by necrosis. Though APAP-induced necrosis shares some features of apoptosis induced by the intrinsic pathway, apoptotic cell death in this context was ruled out due to the absence of caspase activation and lack of protection by caspase inhibitors and missing morphological characteristics of apoptotic cells. Deeper mechanistic understanding of the cell death process after APAP in recent years have now revealed that cells die by programmed necrosis and apoptosis is not a relevant mode of cell death in this context. Hence it is alarming to note that an increasing number of studies are being published purporting to indicate that APAP induces apoptotic cell death. These papers broadly measure “apoptotic markers” with questionable specificity such as Bax, Bcl-2 and caspase-3 protein expression, or use the TUNEL assay as basis for the conclusion that there is apoptosis after APAP overdose. The misguided use of these apoptosis parameters in correlative studies without context or scientific rationale confuses the field and threatens to undo decades of careful mechanistic investigation into this topic. This review examines this emerging problem in detail and recommends approaches to correct it.
Relevance for Patients: Hepatotoxicity and acute liver failure caused by an acetaminophen overdose is a serious clinical problem in western countries. Understanding the mode of cell death and the signaling pathways involved is critical for developing new therapeutic approaches. Recent trends to claim that apoptosis is a relevant mode of cell death in acetaminophen.
DOI: http://dx.doi.org/10.18053/jctres.06.202002.002
Author affiliation
Department of Pharmacology, Toxicology & Therapeutics, University of Kansas Medical Center, Kansas City, KS, USA.
*Corresponding author
Hartmut Jaeschke
Department of Pharmacology, Toxicology & Therapeutics, University of Kansas Medical Center, Kansas City, KS, 66160, USA.
Tel. +1 913 588 7969
Email: hjaeschke@kumc.edu
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

