-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 5 Issue 5
Statins as early therapy to mitigate COVID-19 (SARS-CoV-2)-associated ARDS and cytokine storm syndrome – time is of the essence
Narci Teoh*, Geoff Farrell
Teoh and Farrell, J Clin Transl Res 2020; 5(5): 1
Received: April 15, 2020; Revised: April 16, 2020; Accepted: April 17, 2020
Published online: April 18, 2020
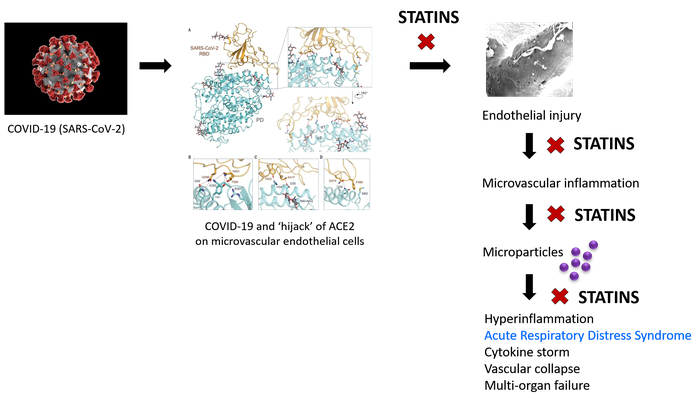
While most COVID-19 infections run a benign course with recovery, the inexorable rise in case fatalities in several countries is grim testimony to the lack of effective therapies to arrest the course of severe infections. Recent observations of Mehta et al. 1 and Matthay et al. 2 have piqued interest in the pathogenic sequence of these severe infections. This leads us to propose an early, safe, and widely-available pharmacological intervention that could be used pre-emptively to avert fatal outcomes in severe SARS-CoV-2-infected patients.
In addition to older age (> 65 years), obesity, and male gender, predictors of COVID-19 mortality are high CRP (> 100 mg/L in fatal vs. 3 mg/L in non-fatal), raised ferritin (mean 1297 ng/mL in non-survivors vs 614 ng/mL in survivors), increased white blood cell count, lymphopenia, and abnormal chest imaging.1,3,4 Hyper-inflammation, cytokine storm, and acute respiratory distress syndromes (ARDS) with profound vascular collapse causing multi-organ failure have been consistently described.1-4 COVID-19-induced hyper-inflammation is characterised by increased production of TNF-α, MIP1-α, IL-6, IL-2, IL-7, and GCSF,1 while alveolar macrophages, CD138+ plasma cells, and T lymphocytes are profuse in bronchoalveolar lavage specimens.5
In animal model studies and patients with ARDS, microparticles (MPs) are released into alveolar and vascular compartments.6 These small vesicles contain membrane and cytosolic proteins, organelles, lipids, and RNA. MPs are shed from different cell types, then interact with other cells to provoke inflammation. In ARDS, released MPs are derived from endothelial and epithelial cells, neutrophils, monocytes, and lymphocytes.6 Notably, lung microvascular endothelial cells express angiotensin-converting enzyme (ACE); endothelial cell-derived ACE+ MPs are prognostic for the development of ARDS in septic patients.7
ACE2 appears to be the cellular receptor for SARS-CoV-2; Yan et al.8 propose that this coronavirus exploits ACE2 in host infection. ACE2 is a type I membrane protein normally expressed on endothelial and epithelial cells in lungs, heart, kidneys, and intestines.8 In the endothelium, ACE2 maintains cellular homeostasis and function.
Enter ‘statins’: these 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors protect the heart, brain, and liver against post-ischaemic injury by mechanisms that transcend lipid-lowering properties. Their mechanisms include stabilization of the vascular endothelium by enhancing endothelial nitric oxide synthase (eNOS) and ACE2 expression.9 If endothelial-derived MPs are central to the pathogenesis of severe COVID-19 infection, there is scientific evidence that early administration of statins offers therapeutic efficacy in an organ system richly endowed with endothelial cells - the liver and its sinusoidal endothelial cells (SECs).
Atorvastatin injected intravenously (5mg/kg body weight) 1 hour before onset of ischemia-reperfusion, a form of microvascular inflammatory liver injury that can complicate shock, conferred ~90% hepatoprotection.9 The mechanisms involve attenuation of systemic MP release, with diminished TNF‐α, IL‐6, MIP‐1α, MCP‐1, GM‐CSF production, decreased thromboxane B2 production, vascular cell adhesion molecule‐1 (VCAM-1) expression, and resultant abrogation of macrophage and neutrophil recruitment. Central to this protective effect of acute statin therapy were increased eNOS expression with enhanced eNOS activity, and protection of SECs observed directly by in vivo liver microcirculation studies.9 Similar hepatoprotection by a dampening of the inflammatory response was observed in a different study using orally administered atorvastatin (5mg/kg body weight).10
If SARS-CoV-2's ‘hijack’ of ACE2 is what initiates microvascular inflammation leading to ARDS and multi-organ failure in fatal COVID-19 infections, statin intervention targeted early in the inflammatory cascade should ameliorate the detrimental pathogenic mechanisms that underlie ARDS, cytokine storm, and vascular collapse.
The utility of statins against ARDS is not new - it was tested in a prospective, randomised, double-blind, placebo-controlled trial dubbed HARP-2.11 In patients with the hyper-inflammatory sub-phenotype of ARDS (high IL-6, soluble TNF-R-1), simvastatin (80 mg/day) administered enterally within 48 hours of lung injury, improved 28- and 90-day survival vs. placebo.12,13 Of note, simvastatin is not the most potent of statins and may not be well absorbed from the gastrointestinal tract of critically ill patients. Statins administered intravenously during coronary ischemia are more effective at decreasing infarct size than oral administration.14 Utilised in other relevant pathophysiological vascular events such as acute coronary syndromes and stroke, statins have been shown to reduce serum CRP, systemic IL-1, IL-6 and TNF‐α release.15,16
We therefore propose a modification of the HARP-2 trial (registration: ISRCTN88244364)11 using intravenous administration of a statin (e.g., atorvastatin, up to 5 mg/kg body weight) early in the course of suspected severe COVID-19 infections, ensued by follow-up intravenous or enteral statin up to 28 days post-randomization.
For entry into such a study, ‘severe’ COVID-19 infection would be defined by the presence of the afore-mentioned poor prognostic factors on presentation and/or within 1 hour of identification of acute lung injury. The primary outcome of such a trial, ‘Statin-HARP-2 plus 1’ (nominally, ‘SHARP-3') would be the number of ventilator-free days. Secondary outcomes would be lung and other organ function, 28- and 90-day mortality, safety, and laboratory analyses. The United Kingdom’s HARP-2 investigators stem from sites now dealing with overwhelming COVID-19 case burdens and high case fatality rates.
We believe ‘SHARP-3’ merits a pilot clinical trial, early and pre-emptively in patients identified at presentation to be ‘at-risk’ of COVID-19 disease severity and mortality. If the preliminary findings prove promising, international multi-centre trials can be rapidly initiated in countries still battling high mortality rates.
References
1. Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-34.
2. Matthay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir Med 2020; published online Mar 20. DOI: 10.1016/S2213-2600(20)30127-2.
3. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; published online Feb 24. DOI: 10.1016/S2213-2600(20)30079-5.
4. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020; published online Mar 26;368:m1091. DOI: 10.1136/bmj.m1091.
5. Giani M, Seminati D, Lucchini A, et al. Exuberant plasmocytosis in bronchoalveolar lavage specimen of the first patient requiring extracorporeal membrane oxygenation for SARS-CoV-2 in Europe. J Thorac Oncol 2020; published online Mar 17. DOI: 10.1016/j.jtho.2020.03.008.
6. McVey M, Tabuchi A, Kuebler WM. Microparticles and acute lung injury. Am J Physiol Lung Cell Mol Physiol 2012;303:L364-81.
7. Takei Y, Yamada M, Saito K, et al. Increase in circulating ACE-positive endothelial microparticles during acute lung injury. Eur Respir J 2019;54:1801188. DOI: 10.1183/13993003.01188-2018.
8. Yan R, Zhang Y, Li Y, et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020;367: 1444–48.
9. Ajamieh H, Farrell GC, McCuskey RS, et al. Acute atorvastatin is hepatoprotective against ischaemia-reperfusion injury in mice by modulating eNOS and microparticle formation. Liver Int 2015;35:2174-86.
10. Ajamieh H, Farrell G, Wong HJ, et al. Atorvastatin protects obese mice against hepatic ischemia-reperfusion injury by Toll-like receptor-4 suppression and endothelial nitric oxide synthase activation. J Gastroenterol Hepatol 2012;27:1353-61.
11. McAuley DF, Laffey JG, O'Kane CM, et al. Simvastatin in the acute respiratory distress syndrome. N Engl J Med 2014;371:1695-703.
12. Calfee CS, Delucchi KL, Sinha P, et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med 2018;6:691-98.
13. Calfee CS, Sinha P. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care 2019;25:12 -20.
14. Mendieta G, Ben-Aicha S, Gutierrez M, et al. Intravenous statin administration during myocardial infarction compared with oral post-infarct administration. J Am Coll Cardiol 2020;75:1386-1402.
15. Gavazzoni M, Gorga E, Derosa G, et al. High-dose atorvastatin versus moderate dose on early vascular protection after ST-elevation myocardial infarction. Drug Des Devel Ther 2017;11:3425-3434.
16. Mohammadkhani N, Gharbi S, Rajanj HF, et al. Statins: Complex outcomes but increasingly helpful treatment options for patients. Eur J Pharmacol 2019;863:172704. DOI: 10.1016/j.ejphar.2019.172704.
DOI: http://dx.doi.org/10.18053/jctres.05.202005.001
Author affiliation
Gastroenterology and Hepatology Unit, Medicine and Surgery Program, The Australian National University, Canberra, Australian Capital Territory, Australia
*Corresponding author
Narci Teoh
Medicine and Surgery Program,
Gastroenterology and Hepatology Unit,
The Australian National University Medical School,
The Australian National University, Acton,
Canberra, ACT 2600,
Australia
Email: narci.teoh@anu.edu.au
Phone: + 61 2 6244 3804
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

