-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 5 Issue 2
Redirection of transfusion waste and by-products for xeno-free research applications
Vanessa Zammit, Mark Farrugia, Byron Baron
Zammit et al., J Clin Transl Res 2019; 5(2): 1
Published on November 9, 2019
Abstract
Background and Aim: Whole blood is processed to derive a red cell concentrate, plasma and buffy coat (from which platelets can be further extracted). Unused plasma and buffy coats are common in most blood establishments and considered a liability. The redirection of these products to xeno-free applications is not complicated or time-consuming and cannot only benefit the research recipients, but also the blood establishment suppliers interested in research collaboration. The aim of this study is to describe a diverse yet by no means exhaustive list of options for preparing blood products for research applications.
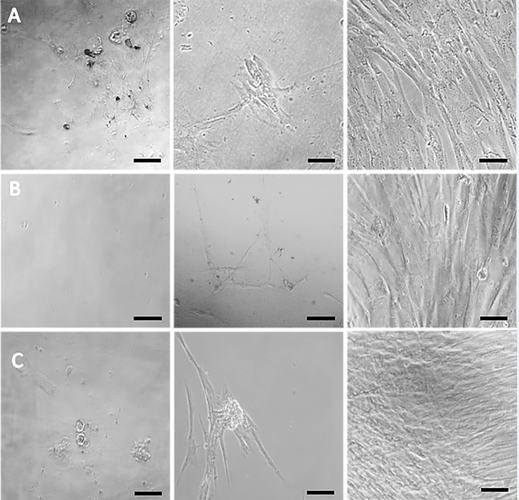
Materials and Methods: Plasma and buffy coats from healthy donors were processed using basic laboratory techniques under aseptic conditions and tested for their ability to support the culture of mesenchymal stem cells in both 2D and 3D cultures using fibrin clots. The white blood cells from the buffy coats were induced by phytoheamagglutinin and CD marker expression was monitored by using qPCR.
Results: All the methods tested for preparing blood products were successful but the applicability to different settings varied greatly with the most successful being the supplementation of DMEM:F12 with 20% cryo-depleted plasma and 0.1 mg/mL platelet lysate, the formation of fibrin clots using a ratio of 3:1 (medium:plasma) and the culturing of white blood cells with 5 µg/mL phytohaemagglutinin.
Conclusions: Using the wastes and by-products of blood establishments for xeno-free cell culturing of stem cells will reduce the reliance on commercially available, ready-made products, increasing the potential for therapeutic stem cell research. Despite the benefits presented both in terms of cost and applications, further characterization and optimization of each blood product for reproducibility of results is required.
Relevance for patients: The availability of low cost xeno-free reagents will speed up therapeutic stem cell research and allow patients to receive treatments of the expected high standards at lower costs.
DOI: http://dx.doi.org/10.18053/jctres.05.201902.001
Author affiliation
1 National Blood Transfusion Service, St. Luke's Hospital, G' Mangia. PTA1010. Malta
2 Centre for Molecular Medicine and Biobanking, University of Malta, Msida, MSD2080. Malta.
*Corresponding author
Byron Baron
Center for Molecular Medicine and Biobanking, Faculty of Medicine and Surgery, University of Malta, Msida, Malta
Tel: 00356 2340 2581
E-mail: byron.baron@um.edu.mt
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

