-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 3 Issue 1
Novel circulating- and imaging-based biomarkers to enhance the mechanism understanding of human drug-induced liver injury
Joanna I Clarke, Nathalie Brillant, Daniel J Antoine
Clarke and Brillant et al., J Clin Transl Res 2017; 3(S1): 199-211
Published online: February 12, 2017
Abstract
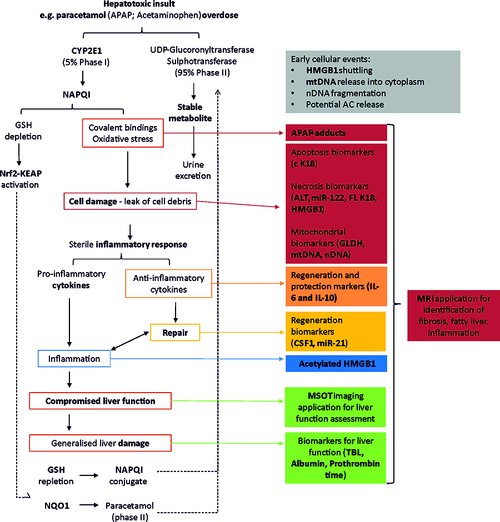
Liver safety biomarkers in current clinical practice are recognized to have certain shortcomings including their representation of general cell death and thus lacking in indicating the specific underlying mechanisms of injury. An informative mechanistic biomarker, or panel of circulating- and imaging- based biomarkers, will allow a more complete understanding of the mechanisms underlying the complex and multi-cellular disease such as drug-induced liver injury; potentially preceding and therefore enabling prediction of disease progression as well as directing appropriate, existing or novel, therapeutic strategies. Several putative liver safety biomarkers are under investigation as discussed throughout this review, informing on a multitude of hepatocellular mechanisms including: early cell death (miR-122), necrosis (HMGB1, K18), apoptosis, (K18), inflammation (HMGB1), mitochondrial damage (GLDH, mtDNA), liver dysfunction (MRI, MSOT) and regeneration (CSF1). These biomarkers also hold translational value to provide important read across between in vitro-in vivo and clinical test systems. However, gaps in our knowledge remain requiring further focused research and the ultimate qualification of key exploratory biomarkers.
DOI: http://dx.doi.org/10.18053/jctres.03.2017S1.005
Author affiliation
Department of Molecular & Clinical Pharmacology, MRC Centre for Drug Safety Science, Institute of Translational Medicine, University of Liverpool, Liverpool, United Kingdom
*Corresponding author
Daniel J Antoine
MRC Centre for Drug Safety Science, Molecular & Clinical Pharmacology, The University of Liverpool Sherrington Buildings, Ashton Street, Liverpool, L69 3GE, United Kingdom
Tel: +44 151 795 5460
Email: d.antoine@liverpool.ac.uk
Handling editors
Hartmut Jaeschke, Anup Ramachandran
Department of Pharmacology, Toxicology & Therapeutics, Kansas University Medical Center, University of Kansas, Kansas City, KA, United States

