-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 2 Issue 3
Hemoporfin-mediated photodynamic therapy on normal vasculature: implications for phototherapy of port-wine stain birthmarks
Wesley J. Moy, Gang Ma, Kristen M. Kelly, Bernard Choi
Moy et al., J Clin Transl Res 2016; 2(3): 107-111
Published online: 3 September, 2016
Abstract
Background: Port-wine stain (PWS) birthmarks currently are treated using a pulsed dye laser (PDL) combined with transient cooling of the epidermis. PDL treatment protocols utilize short pulses of light (585 or 595nm wavelength) to heat selectively the microvasculature due to absorption by intravascular hemoglobin. Although most patients respond to PDL therapy, few experience complete removal of the PWS. An alternate treatment option to PDL therapy of PWS is photodynamic therapy (PDT). Research groups have reported on various photosensitizers for PDT of PWS, including Hemoporfin, Benzoporphyrin Derivative monoacid ring A, and talaporfin sodium.
Aim: Our aim was to evaluate, with an established preclinical in-vivo model, the efficacy of photodynamic therapy (PDT) with Hemoporfin to achieve persistent vascular shutdown.
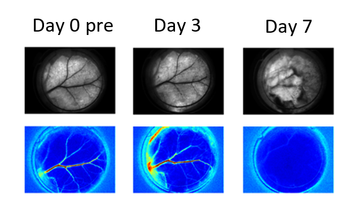
Methods: To monitor the microvasculature, a dorsal window chamber was surgically installed on 24 adult mice. The PDT excitation source emitted 150mW of 532nm light, with an irradiance of 100mW/cm2. A retroorbital injection of Hemoporfin (2 mg/kg) was performed to deliver the drug into the bloodstream. Laser irradiation was initiated immediately after injection. To monitor blood-flow dynamics in response to PDT, we used laser speckle imaging. We employed a dose–response experimental design to study the efficacy of Hemoporfin-mediated PDT to achieve persistent vascular shutdown observed on Day 7 after PDT.
Results: We observed four general hemodynamic responses to PDT: (1) At low radiant exposures, we did not observe any persistent vascular shutdown; (2) at intermediate radiant exposures, we observed delayed vascular shutdown effect with significant change to the vascular structure; (3) at intermediate radiant exposures, we observed an acute vascular shutdown effect with gradual restoration of blood flow and no significant changes to the vascular structure; and (4) at high radiant exposures, we observed acute vascular shutdown that persisted during the entire 7-day monitoring period, with no change in vascular structure. With light dose–response analysis, we estimated a characteristic radiant exposure of 359 J/cm2 that was required to achieve persistent vascular shutdown observed on Day 7 after PDT.
Conclusions: The experimental data collectively suggest that Hemoporfin-mediated PDT can achieve persistent vascular shutdown of normal microvasculature. However, compared with our previous data using Talaporfin Sodium as photosensitizer, Hemoporfin-mediated PDT is less efficient and requires a considerably longer (~four times) irradiation time.
Relevance for patients: Patients with PWS lesions may benefit from the advantages that PDT potentially offers over conventional PDL therapy. PDT potentially is safer for patients of all skin types and more effective at treatment of recalcitrant lesions. Although clinical data suggest that Hemoporfin-mediated PDT is a promising alternative to PDL therapy, our results suggest that additional study of other photosensitizers is warranted.
DOI: http://dx.doi.org/10.18053/jctres.02.201603.003
Author affiliation
1 Beckman Laser Institute and Medical Clinic, University of California, Irvine, CA, United States
2 Department of Biomedical Engineering, University of California, Irvine, CA, United States
3 Department of Surgery, Shanghai Ninth People’s Hospital, Shanghai, China
4 Department of Dermatology, University of California, Irvine, CA, United States
5 Department of Surgery, University of California, Irvine, CA, United States
6 Edwards Lifesciences Center for Advanced Cardiovascular Technology, University of California, Irvine, CA, United States
*Corresponding author:
Bernard Choi
Beckman Laser Institute and Medical Clinic, University of Carlifornia, 1002 Health Sciences Road East, Irvine, CA 92612, United States
Tel: +1 949 824-9491, Fax: +1 949 824-6969
E-mail: choib@uci.edu
Handling editor:
Michal Heger
Department of Experimental Surgery, Academic Medical Center, University of Amsterdam, the Netherlands

