-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Author guidelines
Synopsis
• Double blind peer review process
• Article types: original research, reviews, technical reports, medical hypotheses, commissioned articles, special issue articles, editorials, and translated articles from non-English language journals
• Your paper, your way; no formatting rules until acceptance
• No restrictions on word count, figures, tables, or references
• Unrestricted online supplemental information
• Publication of articles reporting negative research results
Publishing fees
An article processing charge (APC) of USD 1000 applies to papers accepted after peer review. Please find more information here.
Manuscript preparation
Manuscripts are prepared according to the ‘your paper, your way’ style in terms of formatting. However, your submission must contain:
1. A separate cover page with the title, list of authors, affiliation(s) of all authors, email address of all authors, and the full address and contact information of the corresponding author. This cover page must be uploaded as a separate document in Editorial Manager.
2. A cover page containing only the title as part of the main manuscript. This version will be sent to reviewers for a double-blind evaluation of your work.
3. A graphical abstract, preferably embedded in the main manuscript.
4. An abstract and keywords.
5. Manuscript text, figures, tables, and references; preferably all embedded in the main manuscript.
Although the authors are free to structure the manuscript as they please, it is recommended to follow the downloadable template provided by JCTR for original research papers.docx (95.4 KB) and review papers.docx (95.0 KB) . These exemplary manuscript templates were designed such that your work is easy to follow by readers and reviewers, i.e., the people who decide on the value and fate of your work. For clarity and referencing purposes, the numbering of sections and subsections is encouraged. JCTR has also provided a downloadable template for the cover page, a cover letter (not required for submission), and an examplary rebuttal.pdf (42.5 KB) that authors are welcome to use.
JCTR has no restrictions on word count, the number of figures and tables, or references. However, it is strongly advised to proportion the amount of text in the main manuscript to the amount of available data and the essence of the study. Data that is clearly deducible from the figures and tables should not be repeated in the text. There is ample room in the online supplement to present second-order information, which may pertain to any part of the main manuscript. For the sake of reproducibility and clarity, JCTR strongly encourages authors to report all the data of the study and to provide a detailed description of the methods. However, authors are requested to strategically allocate primary data to the main manuscript and secondary data to the online supplemental information.
JCTR has proofreading and illustration services available to optimize your manuscript.
The ‘your paper, your way’ style applies to initial submissions and resubmissions. Upon acceptance of the manuscript, the authors are required to format the manuscript according to the JCTR formatting rules.
Manuscripts must be submitted through the Editorial Manager system.
In order to increase the chances that an article is sent out for review, JCTR encourages authors to understand how manuscripts are handled.
Authorship
Authorship should be limited to people who have contributed substantially to the work. The authorship in JCTR articles should be defined according to the authorship criteria of International Committe of Medical Journal Editors (ICMJE) recommendation. The criteria are as follows:
(1) Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and
(2) Drafting the work or revising it critically for important intellectual content; and
(3) Final approval of the version to be published; and
(4) Agreement to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The corresponding author(s) should be clearly indicated for all manuscripts submitted. The corresponding author(s) is/are responsible for:
(1) obtaining permission from all the authors mentioned in the manuscript; and
(2) ensuring adherence to all editorial and submission policies; and
(3) making communications and actions that may be necessary after publication; and
(4) including written permission from the authors of the work concerned for any mention of any unpublished material included in the manuscript, e.g., data from manuscripts-in-press, personal communication, or work in preparation.
Changes to authorship
The authors should carefully check the list and order of authors before submitting their manuscripts. The editorial office considers the authorship list to be definitive at the time the original submission is received.
Any addition, deletion, or rearrangement of author names in the authorship list should be made only before the manuscript is accepted for publication. The corresponding author should justify the reasons for the change in the authorship list and the proof of written confirmation from all authors (including the existing authors, author(s) to be added and/or removed) agreeing with such a change in a signed letter attached to an email sent to the editorial office for evaluation.
The requests for authorship changes need to be approved in writing by the editorial office before any changes can be made. Note that the changes to authorship should still be in adherence to the authorship criteria of International Committe of Medical Journal Editors (ICMJE) recommendation.
Peer review process
Submitted manuscripts (including Special Issue manuscripts) are handled according to the flow chart below. Authors should take note of the pre-screening procedure by the editorial office to prevent a desk rejection. The items that are prescreened are addressed separately below. It should be noted that the journal actively screens for plagiarism with the assistance of embedded tools in the Editorial Manager system, and the handling editor is in charge of the crosscheck.
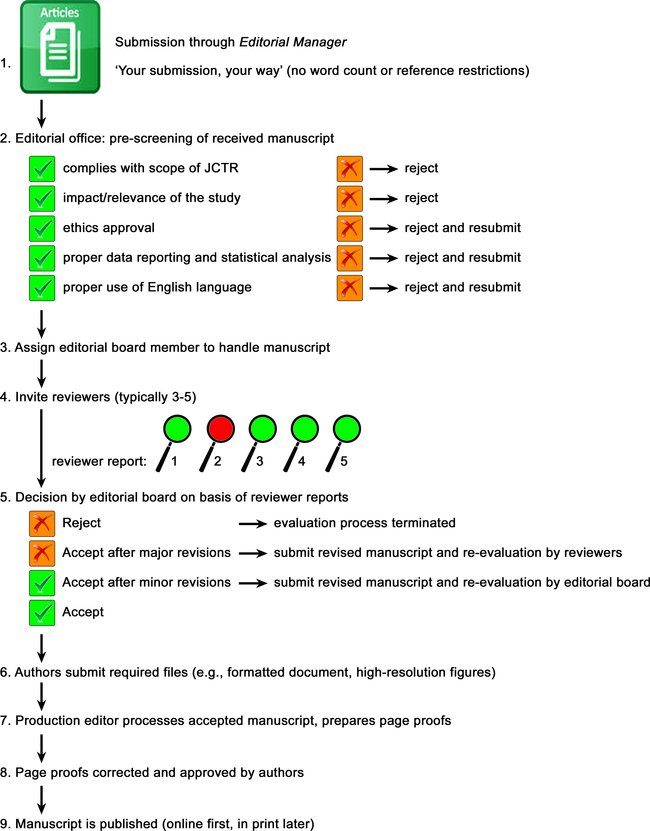
Guest Editors of Special Issues are not able to make decisions regarding their own manuscripts submitted to their Special Issue, as this would constitute a conflict of interest. An Editorial Board member will instead be responsible for decision-making. The Guest Editor will be unable to access the review process except in their role as author. Similarly, Editors-in-Chief or other Editorial Board members are not able to access the review process of their manuscripts except in their role as authors.
Editors' responsibilities:
The handling editors are responsible for monitoring and ensuring the fairness, timeliness, and quality of the peer-review process. Peer review tasks are assigned to external reviewers with the proper expertise. These external reviewers are chosen by the handling editor, to whom the task has been delegated.
Impact and relevance of the study
The impact and relevance of a study are important criteria for JCTR to ensure that the goal of the journal, namely to disseminate information that will ultimately benefit patients, is upheld within the scope of JCTR. Accordingly, a study introducing a novel liquid biopsy technique to diagnose a given cancer with high accuracy will receive priority over a study reporting on an improved method to diagnose cancer with 1% greater accuracy than the gold standard technique. Authors can significantly reduce the chances of a desk rejection decision by explaining the impact and relevance of their study in the manuscript and cover letter.
Conflict of interest policy
Contributing authors are obligated to disclose all possible and actual conflicts of interest related to the submitted work under the ICMJE guidelines.
Reviewers are obligated to contact the managing editor when the manuscript under review constitutes a potential or actual conflict of interest.
Handling editors are to disclose potential and actual conflicts of interest to the managing editor. Reviewers and handling editors will be unassigned from the respective manuscript in the event of a conflict of interest.
Ethics approval
All animal protocols must be performed under institutional guidelines and/or the NIH guide for the care and use of laboratory animals (8th edition, 2011), following approval by the institutional review board. Both should be explicitly confirmed in the materials and methods section. Data should be reported in line with the ARRIVE guidelines for reporting in vivo experiments. A guide for the design and validation of rodent models for biomedical research purposes can be found in Hollingshead MG, J Natl Cancer Inst 2008;100:1500-10.
With respect to clinical studies, only results from clinical trials approved by an institutional review board and entered into an acknowledged clinical trial will be sent out for peer review. The registry identifier should be included in the materials and methods section of the manuscript, and a full link to the registry web page should be cited as a reference. Under the guidelines stipulated by the International Committee of Medical Journal Editors (ICMJE), JCTR recognizes the following clinical trial registries:
• www.anzctr.org.au
• www.clinicaltrials.gov
• www.ISRCTN.org
• www.umin.ac.jp/ctr/index/htm
• www.onderzoekmetmensen.nl/en
• https://eudract.ema.europa.eu/ (new registrations after June 20, 2011)
Authors wishing to submit results from unregistered trials will be requested to make their study protocols available to the editor-in-chief, accompanied by a letter detailing why the study was not registered. The decision to subject these manuscripts to peer review is always at the discretion of the editors.
Preparation guidelines for clinical manuscripts
• Prospective randomized clinical trials must adhere to the CONSORT guidelines and must include a CONSORT flow chart.
• Observational studies should conform to the STROBE statement.
• Additional manuscript type-specific preparation criteria can be found on the website of the EQUATOR network.
For investigations undertaken on human subjects, the manner in which informed consent was obtained from the study participants (i.e., oral or written) should be stated clearly.
The authors should inform the study participants of the purpose(s) of publication, the possible risks and benefits as a result of the experiment, and the patient's right to withhold or withdraw consent. Consent should be obtained from the parent(s) or legal guardian(s) if the study participant is a minor.
The authors are obliged to declare and clearly specify any restrictions on the availability or use of human data in the manuscript.
Human subjects have a right to privacy that should not be violated without informed consent. Identifying information or patient identifiers, including patient names, initials, date of birth, contacts, medical record numbers, hospital numbers, and geographical location, should not be published in written descriptions, photographs, or pedigrees unless the information is essential for scientific purposes and the patient (or parent or guardian) gives written informed consent for publication. Efforts must be made by the authors to at least mask or conceal any identifying information about the patients that appears in writing or within the photograph.
Authors are obliged to explain to the patient if revealing the patient's identity cannot be fully avoided, e.g., an image of an identifiable body part like the face has to be published in the report. The relevant identifying information to be published, e.g., the image, must be shown to the patient, and consent for publication must be obtained for the use of that information in the publication. If the patient dies, then consent must be obtained from the next of kin or legal representative.
Data reporting and statistical analysis
All experimental data reported in the results section must be accompanied by an account of how the data were obtained in respective methods section. qPCR experiments should be reported as per the MIQE guidelines. A practical guide to performing qPCR can be found in Taylor S et al., Methods 2010;50:S1-5.
The authors should perform robust statistical analysis. The book Intuitive Biostatistics by Harvey Motulsky is an accessible and authoritative guide to statistics recommended by JCTR. A synopsis of the book is provided on the GraphPad website. A summary of which statistical test to use can be downloaded here ( JCTR GUIDELINE - Statistics.pdf (19.0 KB) ). For more complex statistical analyses, authors are encouraged to involve a statistician in the data analysis. JCTR has statisticians and epidemiologists on the editorial board who will be asked to validate the statistical methods in selected manuscripts.
English language
Good and effective communication of your data boils down to the proper and correct use of language. Authors are strongly encouraged to carefully proofread the manuscript before submission. A guide for writing scientific papers is made available to contributors who are interested in improving their scientific writing and English skills. JCTR offers proofreading services for authors who need linguistic assistance.
JCTR scope
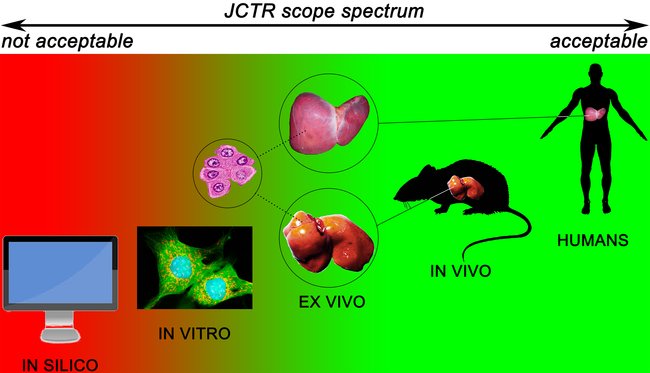
JCTR aims to publish research that ultimately benefits the patient. Accordingly, the published papers should address any clearly defined clinical problem in an attempt to elucidate or (partly) resolve that problem. The research areas covered by JCTR are essentially unrestricted. A non-exhausted list of research areas is provided on the About JCTR page. The main requirement is that original research contains ex vivo, in vivo, and/or clinical data, as depicted below. In vitro, data will be published in exceptional cases. Such exemptions are made for articles addressing, for example, molecular pathways that lie at the basis of a disease, novel biotechnological approaches, e.g., the production of drugs, or new techniques that improve clinical diagnostics and prognostics. Contributions from academic institutions and industry are welcome. Submissions of negative research results are encouraged. In case of doubt, please contact the editor-in-chief.
Copyright policies
This is an open-access article distributed under the Creative Commons Attribution License (CC BY-NC 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Copyright in any article published by the Journal of Clinical and Translational Research is retained by the author(s). The authors grant the journal a license to publish the article and identify itself as the original publisher. Authors also grant any third party the right to use the article freely, provided that it is not used for commercial purposes and its original authors, citation details, and publisher are identified.
Data sharing policies
JCTR adheres to the privacy and data-sharing policies established by the publisher. Find the details here.

