-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 9 Issue 5
Appropriate patient selection and overall survival after transarterial radioembolization in colorectal adenocarcinoma liver metastases
Sharmeen Mahmood, Garo Hagopian, Ben Sadeghi, Jeffrey V Kuo, David K Imagawa, Dayantha Fernando, Nadine Abi-Jaoudeh†, Farshid Dayyani*†
Mahmood et al. J Clin Transl Res 2023; 9(5):23-00066
Published online: September 23, 2023
Abstract
Background and aim: The objective of this study was to describe the overall survival (OS) with transarterial radioembolization (TARE) for patients with colorectal adenocarcinoma liver metastases (CRLM) treated at an academic center with a dedicated multidisciplinary liver tumor board (MTB).
Methods: Single institution retrospective study of consecutive patients with CRLM undergoing TARE with mainly Y90 resin spheres between 01/2016-07/2020.
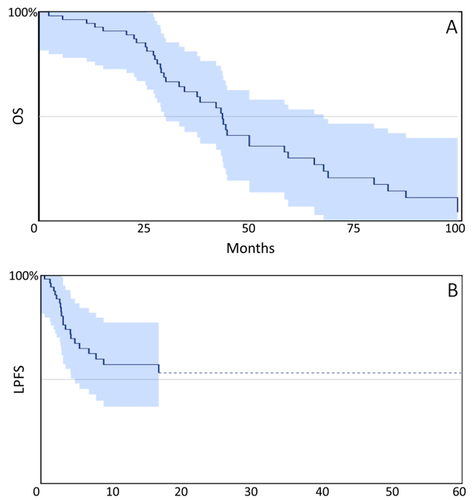
Results: Fifty-five patients were included. Median age was 60 years (range 36-84), 61.8% were female, ECOG 0-1= 90.9%. The median time from diagnosis to first TARE was 16.4 months (1.7-95.6) and 36.4% were treated within the first 12 months of diagnosis. With a median follow-up of at least two years, the median OS from the date of diagnosis and first TARE was 43.2 months (29.5-68.7) and 16.7 months (9.9-35.2), respectively.
Conclusions: The observed OS in this cohort compares favorably to OS reported in contemporary phase 3 trials and might indicate a benefit of TARE with appropriate patient selection at experienced centers with dedicated MTB.
Relevance for Patients: Oncologists treating patients with CRLM should consider referral to a tertiary treatment center with a multidisciplinary team and TARE treatment expertise.
DOI: http://dx.doi.org/10.18053/jctres.09.202305.23-00066
Author affiliation
1. University of California Irvine, Division of Hematology/Oncology, Department of Medicine, Orange, California, United States of America
2. University of California Irvine, Division of Vascular & Interventional Radiology, Department of Radiological Sciences, Orange, California, United States of America
3. University of California Irvine, Department of Radiation Oncology Orange, California, United States of America
4. University of California Irvine, Division of Hepatobiliary Surgery, Department of Surgery, Orange, California, United States of America
†Contributed equally
*Corresponding author
Farshid Dayyani
University of California Irvine, Department of Medicine/Division of Hematology/Oncology, 200 S Manchester Ave, Orange, California (CA) 92868, United States of America.
Tel: +1 714 456 8161
Fax: +1 714 456 2242
Email: fdayyani@hs.uci.edu
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Chemistry, Utrecht University, Utrecht, the Netherlands
Department of Pathology, Erasmus Medical Center, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

