-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 2 Issue 4
Consent2Share: an integrated broad consenting process for re-contacting potential study subjects
Peter Iafrate, Gloria P Lipori, Christopher A Harle, David R Nelson, Timothy J Barnash, Patricia T Leebove, Kathleen A Adams, Debbi Montgomery
Iafrate et al., J Clin Transl Res 2016; 2(4): 113-122
Published online: 10 October, 2016
Abstract
Background and Aim: Obtaining sufficient subjects into research studies is an ongoing barrier to conducting clinical research. Privacy rules add to the complexity of identifying qualified study subjects. The process described facilitates consent of patients coming to their clinically scheduled appointments who are asked to consent to having researchers review their electronic medical records (EHR), and if they meet study criteria for future research, being contacted by those researchers and asked if they wish to be involved in a research project.
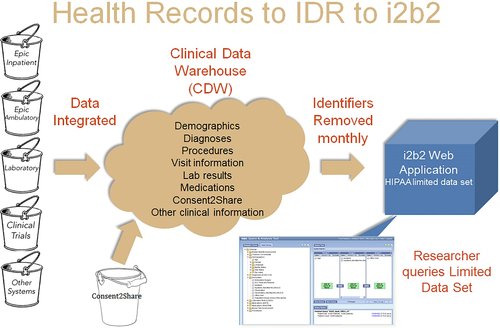
Methods: An interdisciplinary group representing the Institutional Review Board (IRB), Information Technology (IT), Hospital, University and Research developed an initial paper then electronic method to consent all patients attending a medical subspecialty clinic. All consent data are integrated to the EHR to facilitate linking to clinical information.
Results: Although the paper consenting method resulted in over an 80% “yes” rate of consent, it was complicated by significant procedural challenges which prevented scalability. Revising the process has resulted in nearly 28,000 patients consenting in a 3 year period and in 20 IRB approved protocols using subjects who agreed to Consent2Share.
Conclusions: A multi-disciplinary effort is essential to develop a successful electronic based, integrated process to assist investigators and patients to facilitate study subject accrual.
Relevance for patients: Consent2Share more efficiently assists researchers in identifying and contacting potential study subjects that meet entrance criteria. The process provides a model to comply with the proposed Notice of Public Rule Making (NPRM) where institutions will be strongly encouraged to develop broad research consent procedures.
DOI: http://dx.doi.org/10.18053/jctres.02.201604.001
Author affiliation
1 Institutional Review Board, University of Florida, Gainesville, Florida, United States
2 Office of the Chief Data Officer, University of Florida Health, Gainesville, Florida, United States
3 Department of Health Policy and Management, Indiana University, Indianapolis, Indiana, United States
4 Department of Medicine, University of Florida, Gainesville, Florida, United States
5 Practice Management Applications, UF Health Physicians, University of Florida, Gainesville, Florida, United States
6 Medical Specialties and Transplant Clinic, UF Health Physicians, University of Florida, Gainesville, Florida, United States
7 Information Technology, UF Health, Gainesville, Florida, United States
*Corresponding author:
Peter Iafrate
Institutional Review Board, University of Florida, Gainesville, Florida, United States
Email: iafrate@ufl.edu
Handling editor:
Michal Heger
Department of Experimental Surgery, Academic Medical Center, University of Amsterdam, the Netherlands

