-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 8 Issue 3
Vasopressin versus norepinephrine as the first-line vasopressor in septic shock: A systematic review and meta-analysis
Yub Raj Sedhai, Dhan Bahadur Shrestha*, Pravash Budhathoki, Waqas Memon, Roshan Acharya, Suman Gaire, Nisheem Pokharel, Swojay Maharjan, Ranjit Jasaraj, Amik Sodhi, Dipen Kadariya, Ankush Asija, and Markos G. Kashiouris
Sedhai et al. J Clin Transl Res 2022; 8(3):5
Published online: May 25, 2022
Abstract
Background and aim: Norepinephrine is currently the first-line vasopressor for septic shock. We conducted this meta-analysis to examine the outcomes of adult patients with septic shock who received vasopressin instead of norepinephrine.
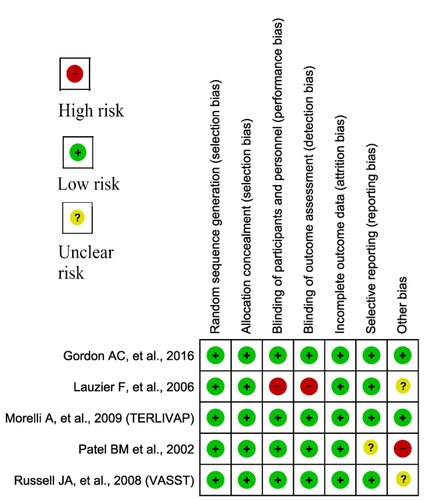
Methods: We selected studies in adults with septic shock that compared the outcomes of patients treated with vasopressin versus norepinephrine. Cochrane ROB 2.0 and the JBI (Joanna Briggs Institute) quality assessment tools were used to assess the risk of bias in RCTs and observational studies. Meta-analysis was conducted using RevMan 5.4.
Results: Eight studies were included in this meta-analysis. There were no significant differences in 28-day mortality rates (OR, 1.07; CI, 0.80-1.44) and ICU mortality (OR, 0.74; CI, 0.21-2.67) between the two groups. Similarly, length of ICU stay, length of hospital stay, mean arterial pressure at 24 hours, urine output at 24 hours, and serious adverse events also did not differ significantly. However, the odds of renal replacement therapy (RRT) requirement in the vasopressin group were substantially lower than in the norepinephrine group (OR, 0.68; CI, 0.47-0.98).
Conclusion: There were no differences in mortality, duration of hospitalization, and adverse effects in adults with septic shock across the two groups. However, the patients treated with vasopressin had lower chances of requiring RRT.
Relevance for patients: Vasopressin use as the first-line vasopressor in septic shock showed a significant reduction in RRT though there were no significant differences in terms of mortality and other adverse events. Therefore, vasopressin can be considered as a first-line vasopressor in septic shock patients with other risk factors which may contribute to renal failure requiring RRT.
DOI: http://dx.doi.org/10.18053/jctres.08.202203.005
Author affilation
1. Department of Internal Medicine, Division of Hospital Medicine, Virginia Commonwealth University, School of Medicine, Richmond, VA, USA
2. Department of Internal Medicine, Mount Sinai Hospital, Chicago, IL, USA
3. Department of Internal Medicine, Bronxcare Health System, Bronx, NY, USA
4. Department of Internal Medicine, Division of Nephrology, University of Virginia School of Medicine, Charlottesville, VA, USA
5. Department of Internal Medicine, Cape Fear Valley Medical Center, 1638 Owen Dr, Fayetteville, NC, USA
6. Department of Emergency Medicine, Palpa Hospital, Palpa, Nepal
7. Department of Emergency Medicine, KIST Medical College, Lalitpur, Nepal
8. Nepalese Army Institute of Health Sciences, Kathmandu, Nepal
9. Department of Internal Medicine, Division of Pulmonary Disease and Critical Care Medicine, University of Wisconsin, Madison, WI
10. Attending Physician, Pulmonary Disease and Critical Care Medicine, Independent Practitioner
11. Department of Internal Medicine, West Virginia University, Morgan Town, WV, USA
12. Department of Internal Medicine, Division of Pulmonary Disease and Critical Care Medicine, VCU School of Medicine, Richmond, VA, USA
*Corresponding author
Dhan Bahadur Shrestha
Department of Internal Medicine, Mount Sinai Hospital, Chicago, IL, USA
Email: medhan75@gmail.com
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

