-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 7 issue 4
Immunotherapy in MSI metastatic colorectal cancer: current status and future perspectives
Rodrigo Motta, Santiago Cabezas-Camarero, Cesar Torres-Mattos, Alejandro Riquelme, Ana Calle, Alejandro Figueroa, Miguel J. Sotelo*
Motta et al. J Clin Transl Res 2021; 7(4):16
Published online: August 4, 2021
Abstract
Background: Colorectal cancer is one of the most frequent and deadly malignancies worldwide. This specific pathology is composed of various molecular entities, with distinct immunological phenotypes. In addition to KRAS, NRAS and BRAF mutation status, other druggable alterations such as those in HER2, MET, NTRK, ALK, and ROS1 have been identified in recent years offering new therapeutic options for some patients with colorectal cancer.
Aim: This review will focus on the molecular biology, immunological fingerprints, and current clinical evidence for the use of immunotherapy in patients with colorectal cancer.
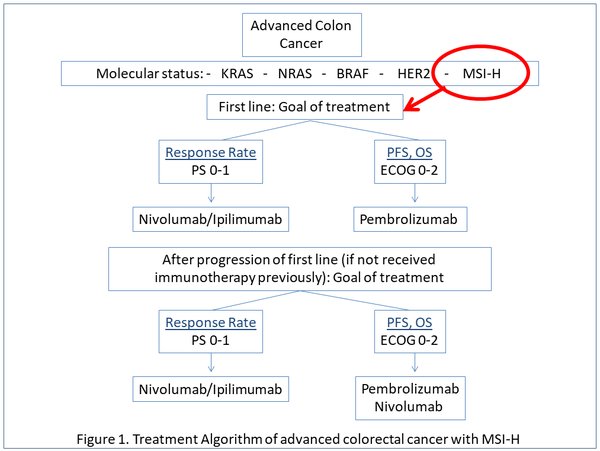
Relevance for patients: High microsatellite instability (MSI-H) and mutations in mismatch repair genes constitute a new molecular entity within colorectal cancer, which is characterized by a high mutational and neoantigen burden, frequent immune cell infiltration, and where immune checkpoint inhibitors have shown high response and survival rates compared to microsatellite stable (MSS) tumors. Indeed, the approval of pembrolizumab in MSI-H tumors was the first agnostic FDA approval in solid tumors. While monotherapy with anti-PD-1 agents achieves objective response rates (ORR) of around 30% and 1-year overall survival (OS) rates of 76%, anti-PD1 and anti-CTLA4 combinations achieve a 55% ORR and a 1-year OS rate of 85%. Several ongoing trials are evaluating the use of different immunotherapy combinations, both in the advanced and early settings and in MSI-h and MSS colorectal cancers.
DOI: http://dx.doi.org/10.18053/jctres.07.202104.016
Author affiliation
1. Department of Medical Oncology, Centro Oncologico Aliada; Lima, Peru
2. Functional Unit of Health Technology, Instituto Nacional de Enfermedades Neoplasicas; Lima, Peru
3. Department of Medical Oncology, Hospital Universitario Clínico San Carlos; Madrid, Spain
4. Department of Medical Oncology, Hospital Nacional Guillermo Almenara Irigoyen; Lima, Peru
5. Oncological Research Unit, Clínica San Gabriel, Lima, Peru.
6. Department of Medical Oncology, Hospital Universitario Infanta Cristina; Madrid, Spain
7. Department of Medical Oncology, Hospital María Auxiliadora; Lima, Peru.
8. Department of Medical Oncology, Hospital Nacional Edgardo Rebagliati Martins; Lima, Peru
*Corresponding author
Miguel J. Sotelo
Department of Medical Oncology, Hospital María Auxiliadora; Centro Oncológico Aliada; Oncological Research Unit, Clínica San Gabriel; Avda. Miguel Iglesias 968, San Juan de Miraflores, Lima 15801, Peru.
Email: miguel.sotelo.lezama@gmail.com
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

