-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 7 issue 3
Mutations of METTL3 predict response to neoadjuvant chemotherapy in muscle-invasive bladder cancer
Zhao Yang#,*, Zongyi Shen#, Di Jin#, Nan Zhang, Yue Wang, Wanjun Lei, Zhiming Zhang, Haige Chen, Faiza Naz, Lida Xu, Lei Wang, Shihui Wang, Xin Su, Changyuan Yu*, Chong Li
Yang et al. J Clin Transl Res 2021; 7(3):9
Published online: June 5, 2021
Abstract
Background and aim: Neoadjuvant chemotherapy (NAC) followed by radical cystectomy is the current gold standard treatment for muscle-invasive urothelial bladder cancer (MIBC). Nonetheless, some MIBC patients showed limited pathological response after NAC. Herein, we used whole-exome sequencing to identify genetic mutations in MIBC that can predict NAC response.
Methods: 40 MIBC patients were enrolled in this study, in which 33 were successfully examined by whole-exome sequencing and Sanger sequencing in the discovery cohort (n = 13) and the validation cohort (n = 20), respectively. ANNOVAR software was used to identify the potential mutations based on the data of whole-exome sequencing. Additionally, tumor-specific somatic mutations including single nucleotide variants (SNVs) and indels were called with the muTECT and Strelka softwares. The mutational analysis of specific genes was carried out based on the data from cBioPortal for Cancer Genomics.
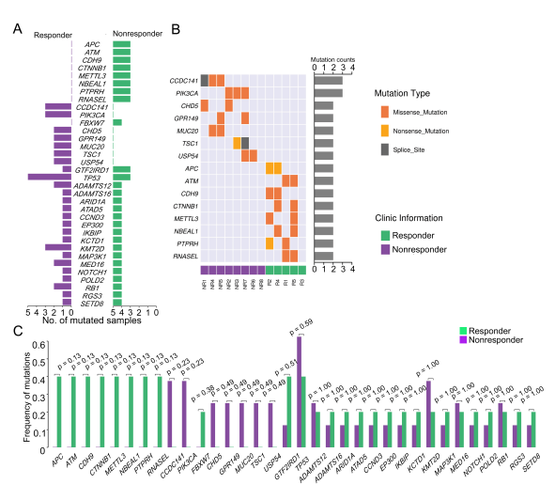
Results: In the discovery cohort, the mutation frequencies of TP53, MED16, DRC7, CEND1, ATAD5, SETD8 and PIK3CA were significantly higher in 13 MIBC patients. Specifically, the presence of somatic mutations of APC, ATM, CDH9, CTNNB1, METTL3, NBEAL1, PTPRH, RNASEL and FBXW7 in NAC responder signifies that these mutations were potential predictors of pathological response to NAC. Furthermore, somatic mutations of CCDC141, PIK3CA, CHD5, GPR149, MUC20, TSC1 and USP54 were exclusively identified in NAC nonresponders, suggesting that these mutations may participate in the process of NAC resistance. In the validation cohort, the somatic mutations of CDH9, METTL3 and PTPRH were significantly enriched in NAC responders while the somatic mutation of CCDC141 was significantly enriched in NAC nonresponders. Furthermore, survival analysis revealed that the patients expressing mutated METTL3 have a longer overall survival and disease- or progression-free survival than the patients acquiring wild-type METTL3.
Conclusion: The somatic mutation of METTL3 can be a potential predictive biomarker of NAC response in MIBC patients.
Relevance for patients: MIBC patients bearing mutated METTL3 display a pathological response to NAC and have a significantly longer overall survival or disease/progression-free survival as compared to the patients bearing wild-type METTL3. Thus, the somatic mutation of METTL3 is a potential biomarker for predicting response to NAC in MIBC patients, assisting doctors in making the clinical decision.
DOI:http://dx.doi.org/10.18053/jctres.07.202103.009
Author affiliation
1. College of Life Science and Technology, Beijing University of Chemical Technology, Beijing 100029, China
2. College of Life Science, Key Laboratory of Protection and Utilization of Biological Resources in Tarim Basin of Xinjiang Production and Construction Corps, Tarim University, Alar 843300, Xinjiang, China
3. Department of Urology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China
4. Cancer Research Department, Novogene Bioinformatics Institute, Beijing 100016, China
5. Core Facility for Protein Research, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China
#These authors contributed equally to this work.
*Corresponding author
Zhao Yang
College of Life Science and Technology, Beijing University of Chemical Technology, Beijing 100029, China.
Phone: +86-10-64421335
E-mail: yangzhao@mail.buct.edu.cn
Changyuan Yu
College of Life Science and Technology, Beijing University of Chemical Technology, Beijing 100029, China.
Phone: +86-10-64451781
Fax: +86-10-64421335
E-mail: yucy@mail.buct.edu.cn
Chong Li
Core Facility for Protein Research, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China.
Phone: +86-10-64888424
Fax: +86-10-64888424
E-mail: lichong@moon.ibp.ac.cn
Handling editor:
Michal Heger
Department of Pharmaceutics, Utrecht University, the Netherlands
Department of Pharmaceutics, Jiaxing University Medical College, Zhejiang, China

