-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 5 Issue 1
Site-specific pharmaco-laser therapy: a novel treatment modality for refractory port wine stains
M. Ingmar van Raath, Jojanneke E. van Amesfoort, Martin Hermann, Yasin Ince, Maurice J. Zwart, Agustina V. Echague, Yan Chen, Baoyue Ding, Xuan Huang, Gert Storm, Michal Heger
van Raath et al., J Clin Transl Res 2019; 5(1): 2
Published online: May 1, 2019
Abstract
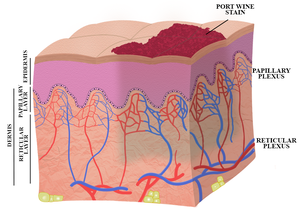
Despite extensive efforts to optimize laser therapy, i.e., the current gold standard treatment, a majority of port wine stain patients responds suboptimally to laser therapy. This paper describes the niceties of a novel port wine stain treatment modality termed site-specific pharmaco-laser therapy (SSPLT). In contrast to the classic approach of enhancing the extent of intravascular photocoagulation (the photothermal response), SSPLT focuses on optimization of post-irradiation thrombus formation (i.e., the hemodynamic response) by combining conventional laser therapy with the administration of thermosensitive drug delivery systems that encapsulate prothrombotic and antifibrinolytic drugs. The aim is to instill complete lumenal occlusion of target vessels, which has been linked to optimal port wine stain blanching.
Relevance for patients: Current treatment options for port wine stain patients are limited in efficacy. Novel therapeutic modalities are needed to more effectively treat patients with recalcitrant port wine stains. SSPLT is an experimental-stage treatment modality that could serve as an adjuvant to pulsed dye laser therapy for a select group of patients whose port wine stain is ill-responsive to standard treatment. The expected clinical result of SSPLT is improved lesional blanching.
DOI: http://dx.doi.org/10.18053/jctres.05.201901.002
Author affiliation
1 Department of Experimental Surgery, Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands
2 Department of Anesthesiology and Critical Care Medicine, Medical University Innsbruck, Innsbruck, Austria
3 Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, MA, USA
4 Department of Clinical Medicine, College of Medicine, Jiaxing University, Jiaxing, Zhejiang, PR China
5 Department of Pharmaceutics, College of Medicine, Jiaxing University, Jiaxing, Zhejiang, PR China
6 Department of Pharmaceutics, Utrecht Institute for Pharmaceutical Sciences, Utrecht University, Utrecht, the Netherlands
7 Department of Controlled Drug Delivery, MIRA Institute for Biomedical Technology and Technical Medicine, University of Twente, Enschede, the Netherlands
8 Membrane Biochemistry and Biophysics, Bijvoet Center for Biomolecular Research, Institute of Biomembranes, Utrecht University, Utrecht, the Netherlands
*Corresponding author:
Michal Heger
Department of Pharmaceutics, College of Medicine, Jiaxing University, Jiaxing, Zhejiang, PR China
Email: m.heger@jctres.com
Membrane Biochemistry & Biophysics, Department of Chemistry, Bijvoet Center for Biomolecular Research & Institute of Biomembranes, Utrecht University, the Netherlands
Email: m.heger@uu.nl
Tel: +31-30-2533966
Handling editor:
Rowan van Golen
Gastroenterology and Hepatology, Leiden University Medical Center, the Netherlands

