-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 3 Issue 2
Importance of intellectual property generated by biomedical research at universities and academic hospitals
Joris J. Heus, Elmar S. de Pauw, Mirjam Leloux, Margherita Morpurgo, Michael R Hamblin, Michal Heger
Heus et al., J Clin Transl Res, 2017, 3(2): 5
Published online: May 24, 2017
Abstract
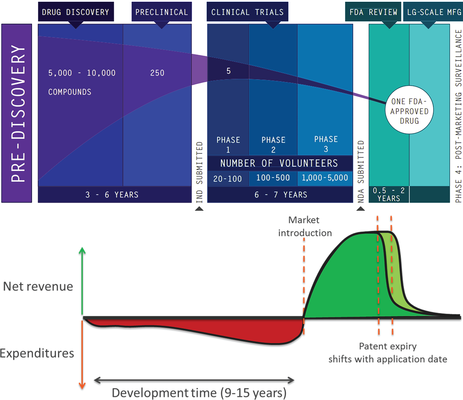
Biomedical research has many different facets. Researchers and clinicians study disease biology and biochemistry to discover novel therapeutic targets, unravel biochemical pathways and identify biomarkers to improve diagnosis, or devise new approaches to clinically manage diseases more effectively. In all instances, the overall goal of biomedical research is to ensure that results thereof (such as a therapy, a device, or a method which may be broadly referred to as “inventions”) are clinically implemented. The development and implementation of an invention can be arduous and very costly. Historically, it has proven to be crucial to protect intellectual property (IP) rights to an invention (i.e., a patent) to ensure that companies can obtain a fair return on their investment that is needed to develop an academic invention into a product for the benefit of patients. However, the importance of IP is not generally acknowledged among researchers at academic institutions who are active in biomedical research. Therefore this paper aims to (1) raise IP awareness amongst clinical and translational researchers; (2) provide a concise overview of the steps that the patenting trajectory entails; and (3) highlight the importance of IP protection for both the research and the researcher.
Relevance for patients: Adequate patent protection of inventions generated through biomedical research at academic institutions increases the probability that patients will benefit from these inventions, and indirectly enables the funding of clinical studies, mainly by opening up more opportunities for diversified funding (e.g. specific grants aimed at start-ups, pre-seed and seed capital) that otherwise would not be accessible. As a consequence, patented inventions are more likely to become clinically tested and reach the market, providing patients with more treatment options.
DOI: http://dx.doi.org/10.18053/jctres.03.201702.005
Author affiliations
1 Innovation Exchange Amsterdam (IXA) Office AMC, Academic Medical Center, University of Amsterdam, Amsterdam, the Netherlands
2 Innovation Exchange Amsterdam (IXA) Office UvA-HvA, University of Amsterdam, Amsterdam, the Netherlands
3 Department of Pharmaceutical and Pharmacological Sciences, University of Padova, Padova, Italy
4 Technology Transfer Office, University of Padova, Padova, Italy
5 Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, United States
6 Department of Dermatology, Harvard Medical School, Boston, United States
7 Harvard-MIT Division of Health Sciences and Technology, Cambridge, United States
8 Department of Experimental Surgery, Academic Medical Center, University of Amsterdam, Amsterdam, the Netherlands
*Corresponding author
Joris J. Heus
Innovation Exchange Amsterdam Office, Academic Medical Center, University of Amsterdam, Amsterdam, the Netherlands
Tel: +31 (0)20 5667911
Email: j.heus@ixa.nl
Handling editor:
Yao Liu
Department of Membrane Biochemistry and Biophysics, Utrecht University, the Netherlands

