-
Home
-
About JCTR
-
Gold Open Access
-
Issues
-
Editorial board
-
Author guidelines
-
Publication fees
-
Online first
-
Special issues
-
News
-
Publication ethics
-
Partners
-
Submit your manuscript
-
Submit your review report
-
Editorial Office
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. ISSN print: 2382-6533 ISSN online: 2424-810X
Volume 3 Issue 1
Immune mechanisms of idiosyncratic drug-induced liver injury
Alastair Mak, Jack Uetrecht
Mak and Uetrecht, J Clin Transl Res 2017; 3(S1): 145-156
Published online: February 12, 2017
Abstract
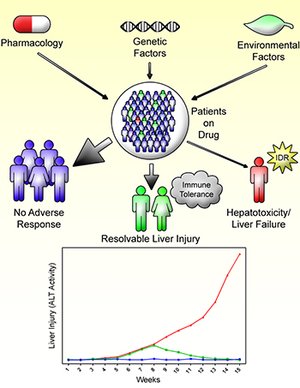
Idiosyncratic drug reactions (IDRs) continue to be an important issue. Specifically, idiosyncratic drug-induced liver injury (IDILI) is the most likely IDR to lead to drug withdrawal, and it accounts for a significant portion of all cases of acute liver failure. In addition, IDRs are unpredictable and their mechanisms are not well understood. There is increasing clinical evidence that most IDILI is immune mediated. Several immune mediated mechanistic hypotheses exist such as the hapten and danger hypothesis; however, they do not completely explain the idiosyn-cratic nature of these reactions. Extensive mechanistic studies are needed to better understand these reactions; however, it is impossible to do controlled experiments in humans, and previous animal models did not properly model IDILI. If IDILI is immune mediated and the major factor preventing liver injury in patients is immune tolerance, then a plausible method to develop an animal model of IDILI would be to impair immune tolerance. This hypothesis has shown promise in developing valid animal models of IDILI as demonstrated by a halothane induced liver injury mouse model developed by depleting myeloid derived suppressor cells (MDSCs), as well as an amodiaquine-, isoniazid- and nevirapine-induced liver injury mouse model developed by impairing immune tolerance by blocking PD-1 and CTLA-4, two immune checkpoint inhibitors. Further characterization and validation of these models is required; however, it is likely that they will make it possible to perform mechanistic studies that have been impossible in the past.
Relevance for patients: Idiosyncratic drug-induced liver injury can be serious leading to liver transplantation or death. Their idiosyncratic nature makes mechanistic studies very difficult. However, with the development of the first animal model that is similar to the liver injury that occurs in humans, it will be possible to study the mechanisms involved. With a better mechanistic understanding it should be possible to test drug candidates and produce safer drugs. In addition, it should be possible to design better treatments when drug-induced liver injury does occur.
DOI: http://dx.doi.org/10.18053/jctres.03.2017S1.001
Author affiliation
Department of Pharmaceutical Sciences, Faculty of Pharmacy, University of Toronto, Toronto, Ontario, Canada
*Corresponding author:
Jack Uetrecht
Leslie Dan Faculty of Pharmacy University of Toronto 144 College Street Toronto, Ontario M5S 3M2
E-mail:jack.uetrecht@uroronto.ca
Tel: +1 416 978 8939
Handling editors:
Hartmut Jaeschke, Anup Ramachandran
Department of Pharmacology, Toxicology & Therapeutics, Kansas University Medical Center, University of Kansas, Kansas City, KA, United States

